
AAV Vector Gene Therapy Testing for Cell and Gene Therapy
Digital PCR for Viral Vector Development, Characterization, and Quantitation
Cell and gene therapies are utilized to treat genetic diseases by introducing genetic material into the patient in order to alter cell function. These therapies can be delivered in vivo using viral vectors modeled on adenoviruses, adeno-associated viruses, or retroviruses, with the goal of either replacing, editing, adding to, or silencing the targeted gene.
The production of sufficient quantities of viral vectors is important in affecting gene transfer. In this step, characterization and quantification become key in viral vector manufacturing for therapies.
Digital PCR is becoming an indispensable tool in the analysis of viral vectors for cell and gene therapy applications. The absolute quantification afforded by digital PCR – as opposed to the relative quantification using qPCR – provides researchers with the ability to precisely quantify and characterize viral vectors.

From sample extraction to analysis for viral vectors
The Nio+ system offers a fast, easy-to-use platform for quantification of genetic targets. In order to detect and quantify multiple genetic targets in a single run, the Nio+ system also offers the highest multiplexing available on the market today. When coupled with a sample preparation platform such as the Maxwell® RSC instruments from Promega, users are provided with a seamless end-to-end workflow – from sample preparation to analysis.
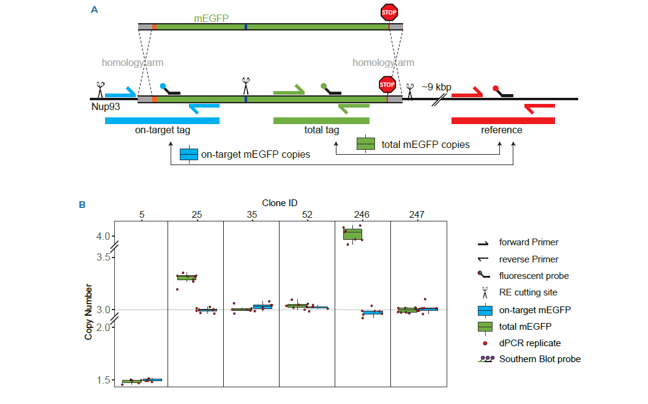
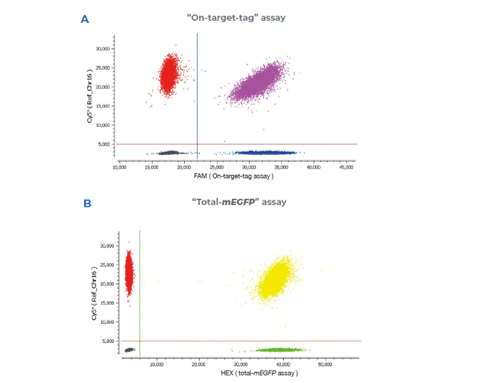
Get absolute quantification for recombinant vector integrity along with precise and sensitive measurement of expression. Data analysis with Crystal Miner software enables straightforward assay optimization through intuitive QC outputs and unique droplet visualization.


Digital PCR in Cell and Gene Therapy
Robust, reproducible, standardized development process
- Recombinant vector integrity
- Viral titer
- Transgene copy number
- Level transgene expression
Scalable production, dependable safety assessment, quality, dosing
- Recombinant vector integrity
- Viral titer
- Transgene copy number
- Residual DNA
Therapeutics efficacy in patients
- Biodistribution
- Longitudinal monitoring
- Response to treatment
Quantification and characterization for cell and gene therapy
Stilla provides digital PCR kits out-of-the-box, as well as custom assay design services in order to help researchers characterize and quantify genetic targets across cell and gene therapy applications.

From custom assay design services to onsite training, Stilla has the knowledge and expertise to help researchers get started on their workflow. Stilla technologies has compiled several statistical tools to help get your started with your digital PCR assays, including:
For Research Use Only. Not for use in diagnostic procedures.

