LIQUID BIOPSY AND ctDNA TESTING WITH CRYSTAL DIGITAL PCR™
Providing sensitivity for patient samples through ctDNA testing
Liquid biopsies are a minimally invasive sampling approach to overcome the heterogeneity of tumors and represent a valuable source of circulating tumor DNA (ctDNA) for oncological biomarker analysis. These samples contain a wealth of genetic information that can be used to inform disease diagnosis, guide therapeutic actions, monitor treatment effectiveness, and detect disease relapse.
The use of ctDNA as a surrogate for tissue DNA has become increasingly popular, and advances in the detection and characterization of ctDNA have enable liquid biopsy assay integration into clinical practice.
ctDNA measurements require a highly sensitive and reliable detection technology to quantify often low-level genetic aberrations within a high background of wild-type sequences, and digital PCR has emerged as a powerful technology for the next-generation analysis of liquid biopsies.

From sample extraction to analysis
The Nio+ system offers a fast, easy-to-use platform for quantification of genetic targets with the highest multiplexing available on the market today. When coupled with a sample preparation platform such as the Maxwell® RSC instruments from Promega, users are provided with a seamless end-to-end workflow – from sample preparation to analysis.

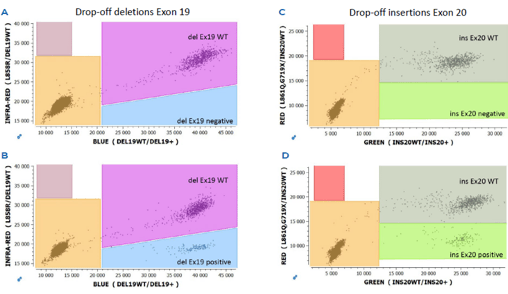
Detect genetic mutations across oncological indications
Stilla provides digital PCR kits out-of-the-box, as well as custom assay design services in order to help researchers detect and quantify genes across multiple cancer indications through a liquid biopsy test.
EGFR mutation detection
ESR1 mutation detection
Other indications

From custom assay design services to onsite training, Stilla has the knowledge and expertise to help researchers get started on their workflow. Stilla technologies has compiled several statistical tools to help get your started with your digital PCR assays, including:
For Research Use Only. Not for use in diagnostic procedures.