Naica® System Compatible Digital PCR Test Kit and Assays
Detect and Quantify Nucleic Acids
Crystal Digital PCR® kits allows researchers to detect and quantify nucleic acids with ready-to-use, pre-designed assays for fully-validated target panels. These digital PCR panel kits are specially developed by Stilla Technologies and its partners. For fully-customizable kits and/or custom assay development, contact our expert services team.

Crystal Digital PCR® Kits for Oncology
EGFR 6-color Crystal Digital PCR® Kit
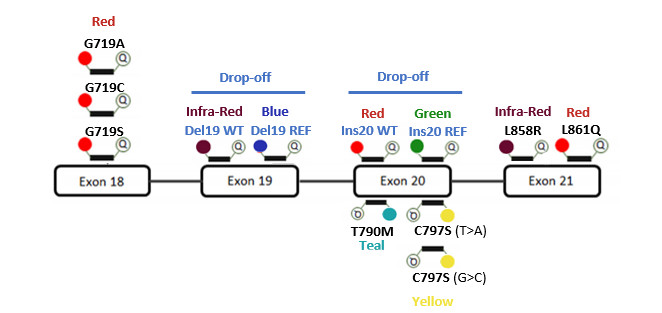
The EGFR 6-color Crystal Digital PCR® Kit allows for the detection and quantification of up to 32 somatic EGFR mutations in exons 18, 19, 20, and 21 associated with non-small cell cancer (NSCLC).
The EGFR 6-color Crystal Digital PCR® Kit provides a fully validated, specific, and ultrasensitive assay for the 6-color naica® and Nio™+ systems. The kit can be used to test cell-free DNA (cfDNA) extracted from plasma samples and genomic DNA isolated from FFPE tissue sections.
The EGFR 6-color Crystal Digital PCR® Kit is a ready-to-use solution comprised of six components, including the high-perfomance naica® multiplex PCR MIX 10X, an optimal primer and probe solution, a set of positive and negative controls, and water for sample dilution.
| Reference | Product Name | Number of Reactions |
|---|---|---|
| R30006 | EGFR 6-color Crystal Digital PCR® Kit | 192 reactions on the Ruby Chip on Nio™+ 48 reactions on the Sapphire Chip on the naica® system |

EGFR 6-color Crystal Digital PCR® Kit technical resources

The IDENTIFY Kit Line
The IDENTIFY digital PCR kit line provides detection for multiplexed mutation panels using fully-validated, specific, and sensitive Crystal Digital PCR™ solutions on the 3-color naica® system for a variety of oncology target panels.
Use Crystal Digital PCR™ to test for mutations across multiple indications using circulating cell-free DNA from liquid biopsies or tumor DNA from solid biopsies.

The IDENTIFY Kit Line
IDNRAS Solution
Detection of NRAS mutations from tumor DNA
IDEGFR Solution
Detection of EGFR mutations in non-small cell lung cancer (NSCLC)
IDKRAS Solution
Detection of KRAS mutations from tumor DNA
IDBRAF Solution
Detection of BRAF mutations at position V600
IDPIK3CA Solution
Detection of PIK3CA mutations from tumor DNA
IDIDH1-2 Solution
Detection of IDH1 and IDH2 mutations from tumor DNA
IDH3F3A Solution
Detection of H3F3A mutations from tumor DNA
IDBRAF-CNS Solution
Detection of BRAF mutations in brain tumors
IDFGFR1-CNS Solution
Detection of FGFR1 mutations in brain tumors
IDTERT Solution
Detection of TERT gene promoter mutations in tumor DNA

Digital PCR Kits for Clinical Screening
iSAFE™ Non-Invasive Prenatal Test Assay
The iSAFE™ Non-Invasive Prenatal Test (NIPT) Assay applies Crystal Digital PCR™ technology on the 6-color naica® system to accurately quantify the copy numbers of chromosome 21, 18, and 13 in maternal blood cell-free DNA (cfDNA). The iSAFE™ NIPT Assay screens for aneuploidy of chromosomes 21 (Down Syndrome), 18 (Edwards Syndrome), and 13 (Patau Syndrome) in singleton pregnancies and allows for fetal fraction calculation and gender determination.
The iSAFE™ NIPT Assay includes optimized reagents to be used in combination with the naica® multiplex PCR MIX to screen for multiple targets in a high-plex and simple workflow on the naica® system.
(The iSAFE™ NIPT Assay does not include the naica® multiplex PCR MIX, which is sold separately.)
The iSAFE™ NIPT Assay is only available in selected regions. The exclusion of commercial NIPT services applies only in the United States and the European Union.

Product flyer
For Research Use Only. Not for use in diagnostic procedures.
Request a Quote
Harness the power of the naica® system and get started with Crystal Digital PCR™ today.